Formulation R&D unit
Thank your for visiting the Medicostech website.
Home  Business Unit&Product Information
Business Unit&Product Information Formulation R&D unit
Formulation R&D unit Research technology
Research technology
 Business Unit&Product Information
Business Unit&Product Information Formulation R&D unit
Formulation R&D unit Research technology
Research technologyscience cosmetics
Liposome
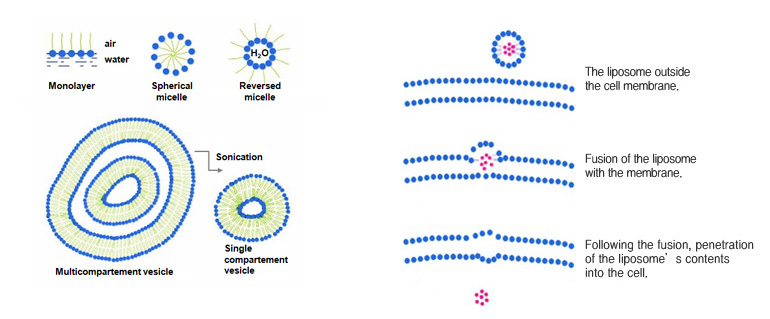
Scattering amphipathic lipids that form lecithin and lamella liquid crystals in water in excess produces small closed vesicle.This is called a liposome.
Vesicles are different from micelles in that they are not stable thermodynamically, and the size and structure could be variable depending on the methods of synthesis.
This structure serves as a capsule and are widely used in pharmaceutics and cosmetics industry.
Liposome is a stabilized structure formed by enveloping hydrophilic compound in a form similar to lipid bilayer of a cell membrane, crystallizing them and then maintain this form in hydrophilic solution in a colloid form.
By keeping the hydrophilic lipid materials which are impossible to penetrate into the skin inside a layer of oil and then incorporating active ingredients into the membrane, a structure similar to biological ell membrane is synthesized.
Liposome composed of a lipid bilayer will infiltrate hydrophilic materials into the dermis through the epidermal intercellular space also composed of lipid bilayer or by directly passing through the cell itself.
Major functions/strengths of liposomes
·Its structure has high affinity for biological membranes and can infiltrate hydrophilic active ingredients deep into the skin.·Little irritation since lecithin and other materials used in emulsification of liposome are natural products.
·Protect unstable drugs that could easily be converted or degraded by stabilizing them and prevent proteins or enzymes from being degraded by macrophages, etc.
·May be transferred to specific organs or tissues by binding compounds that recognize the target cell (Ligands, Antibody etc.)
Emulsion
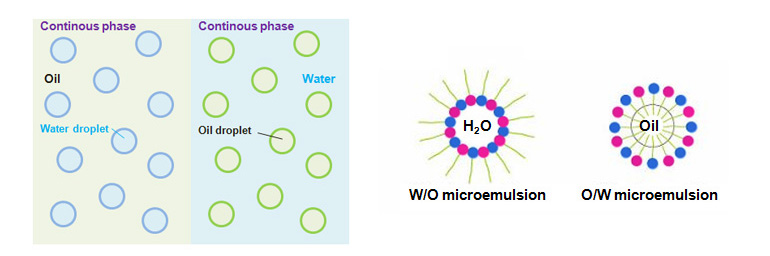
Dispersed system of liquids that are not soluble in each other such as water and oil is called emulsion, and synthesis of such system is called emulsification.Emulsion is a type of dispersion colloid or coarse dispersion system, thermodynamically unstable, and is ultimately separated.
Emulsions are normally white turbid solution, but when the refractive index is constant, emulsions with large droplets can be made into clear solutions.
The biggest problem in emulsion is how to stabilize the emulsions for a long time, and the solution to such problem holds a very important key in manufacture of cosmetics.
As we see from common examples in our daily lives such as milk, hydrophilic dispersion colloids such as gelatin without regards to dispersoids are sometimes called emulsions, and sometimes we do not distinguish between dispersed particles and the demulsified colloids that are the same size as the dispersed particles.
Shaking and mixing two liquids that are not soluble such as water and oil will produce emulsions, but normally these emulsions are unstable that they will separated back into two liquids when undisturbed. In order to stabilize the solution in the emulsified state, it is common to add emulsifying agents
When there is an emulsion of water and oil, there is O/W type emulsion where oil is dispersed in the aqueous solution, and W/O type emulsion where water droplets are dispersed in oil.
To distinguish between these two types, place one drop of emulsion under the microscope and let it come into contact with water or oil.
If the emulsion mixes freely with water, the emulsion is O/W type, and if the emulsion mixes freely with oil, the emulsion is W/O type.